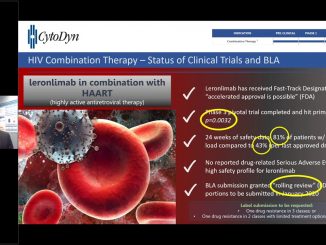
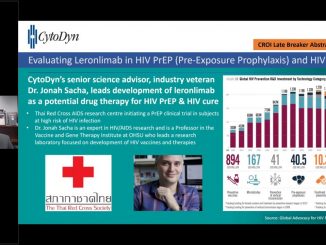
CytoDyn to Hold Conference Call for Updates on Filing Phase 2 Trial with FDA for Treatment of Coronavirus in U.S., Status of BLA, Breakthrough Therapy Designation, Basket Trial for 22 Solid Tumor Cancers and Licensing Opportunities in Several Countries
VANCOUVER, Washington, Feb 28, 2020 — CytoDyn Inc. (otc.qb:CYDY), (“CytoDyn” or the “Company”), a late-stage biotechnology company developing leronlimab (PRO 140), a CCR5 antagonist with the potential for multiple therapeutic indications, announced today that Nader […]